At my college, I had a poster session where individuals are asked to create posters of some research project or work that they did and present them. I decided to create my poster about titanium anodizing. Since the time when I discovered the range of colors obtainable with titanium when it is anodized, I was fascinated by the process. Therefore, I decided to use the entire range of voltages to create the complete anodizing spectrum.
I ran into some electrical problems, however. I could only get a top voltage of 63 volts with nine volt batteries, so I decided to ask my neighbor to loan me his variable transformer. After a couple of fuse blowings from incorrectly connected wires (the strands of the wire were spread out and making undesirable electrical connections), he soldered the wires to the bridge rectifier that I was using. But there were more problems.
I remember learning about RMS voltage and how that 120 volts is not really 120 volts. But I did not realize this at the time so I believed that "70 volts" as marked on the AC voltage side would translate to a peak of 70 volts on the DC side. However, this was only the average; the peak voltage was somewhere around 110 volts, as evidenced by the anodizing color. After several unsuccessful attempts, we placed a motor starter capacitor in series to level out the voltage. It worked much better after that. Read about root mean square on Wikipedia for more information about the mathematics.
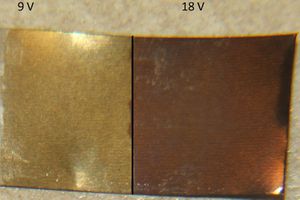
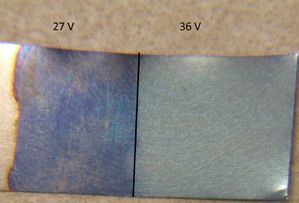
The lower voltages (18 volts and 24 volts) produced saturated colors (dark brown and dark blue, respectively). Nine volts produced a light tan, while 36 volts produced a light blue.
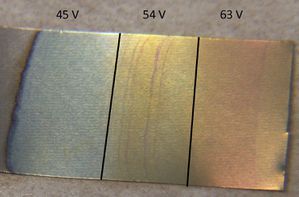
Voltages from 45 to 63 volts produced colors ranging from light green to light yellow to light yellow-pink.
Although light green supposed to be 50 volts while yellow-pink is 90, leaving the piece of titanium in the electrolyte for an extended period of time often makes the voltage seem higher than it really is (e.g. 45 volts makes a 70 volt color).
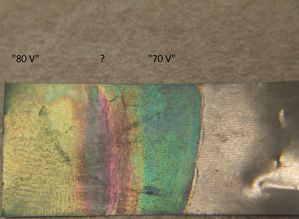
Because of the RMS difficulty mentioned beforehand, "70 volts" made a greenish-blue coloration, while "80 volts" made a lightly greenish coloration. Higher voltages made a whitish surface that was partially permeable to further titanium oxidation. This is when I realized the voltage problem. (I'm not sure why the purplish spot formed in the middle of the titanium.)
Then I added the capacitor to see the real voltage. 55 volts made a lightly greenish surface.
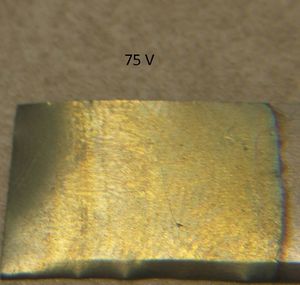
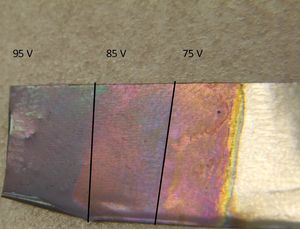
75 volts made an indigo-colored surface. 85 volts was purplish/indigo, while 95 volts was had a light purplish color.
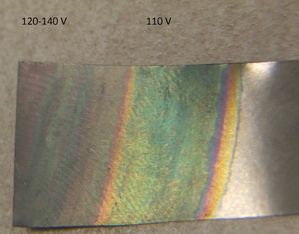
110 volts made a greenish color, while higher voltages made a whitish and faintly iridescent coloration.
Therefore, clean-cut charts about the anodization color when compared to voltage (like
this are rather impossible to make. Extended periods of time in a solution at a particular voltage tends to show a coloration higher than what was expected. Momentary contact results in a lower-voltage color. Therefore, it is best to use a relatively low voltage like 45 volts for the lower colors (and 110 volts for higher colors) and just vary the time that the current is switched on to produce the different color effects.
Niobium, a similar metal, anodizes similarly to titanium. Anodized niobium jewelry is relatively common as it can be very colorful, durable, and hypoallergenic. Here is some anodized niobium.
Here is the poster itself.